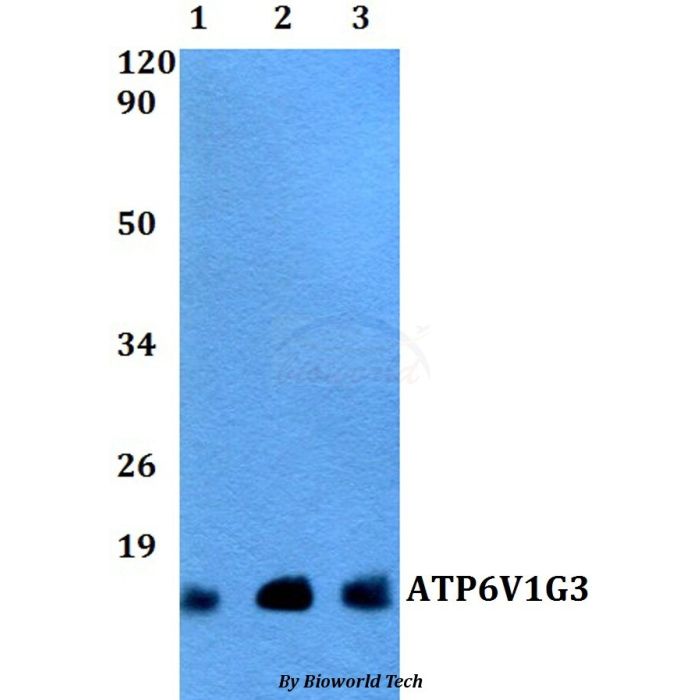
ATP6V1G3 polyclonal, anti-human, mouse, rat
€305.00
In stock
SKU
BS60498
Background:
Vacuolar-type H+-ATPase (V-ATPase) is a multisubunit enzyme responsible for acidification of eukaryotic intracellular organelles. V-ATPases pump protons against an electrochemical gradient, while F-ATPases reverse the process, thereby synthesizing ATP. A peripheral V1 domain, which is responsible for ATP hydrolysis, and a integral V0 domain, which is responsible for proton translocation, compose V-ATPase. Nine subunits (A–H) make up the V1 domain and five subunits (a, d, c, c' and c") make up the V0 domain. Like F-ATPase, V-ATPase most likely operates through a rotary mechanism. In yeast, the V-ATPase G subunit is a soluble subunit that shares homology with the F-ATPase G subunit and may be part of a connection stalk between V1 and V0. The G2 isoform of the G subunit associates with the pore-forming a1C-subunit of L-type calcium channel and aids in proper membrane targeting of the calcium channel. The genes encoding the G1 and G2 V-ATPase subunits map to chromosomes 9q33.1 and 6p21.3, respectively.
Alternative Name:
V-type proton ATPase subunit G 3, V-ATPase subunit G 3, V-ATPase 13 kDa subunit 3, Vacuolar proton pump subunit G 3, ATP6V1G3, ATP6G3
Application Dilution: WB: 1:500~1:1000
Specificity: ATP6V1G3 polyclonal antibody detects endogenous levels of ATP6V1G3 protein.
Immunogen:
A synthetic peptide corresponding to residues in Human ATP6V1G3.
MW: ~ 14 kDa
Swis Prot.: Q96LB4
Purification & Purity:
The antibody was affinity-purified from rabbit antiserum by affinity-chromatography using epitope-specific immunogen and the purity is > 95% (by SDS-PAGE).
Format:
1 mg/ml in Phosphate buffered saline (PBS) with 15 mM sodium azide, approx. pH 7.2.
Storage:
Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze-thaw cycles.
For research use only, not for use in diagnostic procedure.
Vacuolar-type H+-ATPase (V-ATPase) is a multisubunit enzyme responsible for acidification of eukaryotic intracellular organelles. V-ATPases pump protons against an electrochemical gradient, while F-ATPases reverse the process, thereby synthesizing ATP. A peripheral V1 domain, which is responsible for ATP hydrolysis, and a integral V0 domain, which is responsible for proton translocation, compose V-ATPase. Nine subunits (A–H) make up the V1 domain and five subunits (a, d, c, c' and c") make up the V0 domain. Like F-ATPase, V-ATPase most likely operates through a rotary mechanism. In yeast, the V-ATPase G subunit is a soluble subunit that shares homology with the F-ATPase G subunit and may be part of a connection stalk between V1 and V0. The G2 isoform of the G subunit associates with the pore-forming a1C-subunit of L-type calcium channel and aids in proper membrane targeting of the calcium channel. The genes encoding the G1 and G2 V-ATPase subunits map to chromosomes 9q33.1 and 6p21.3, respectively.
Alternative Name:
V-type proton ATPase subunit G 3, V-ATPase subunit G 3, V-ATPase 13 kDa subunit 3, Vacuolar proton pump subunit G 3, ATP6V1G3, ATP6G3
Application Dilution: WB: 1:500~1:1000
Specificity: ATP6V1G3 polyclonal antibody detects endogenous levels of ATP6V1G3 protein.
Immunogen:
A synthetic peptide corresponding to residues in Human ATP6V1G3.
MW: ~ 14 kDa
Swis Prot.: Q96LB4
Purification & Purity:
The antibody was affinity-purified from rabbit antiserum by affinity-chromatography using epitope-specific immunogen and the purity is > 95% (by SDS-PAGE).
Format:
1 mg/ml in Phosphate buffered saline (PBS) with 15 mM sodium azide, approx. pH 7.2.
Storage:
Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze-thaw cycles.
For research use only, not for use in diagnostic procedure.
| Is Featured? | No |
|---|
Write Your Own Review