FluoroType® SARS-CoV-2varID Q
€0.00
In stock
SKU
HL-1885892
Catalog Number: HL-1885892, Size: 96 reactions
Multiplex PCR assay for quantitative detection of SARS-CoV-2 and identification of SARS-CoV-2 S gene mutations
Brochure, Questions? Contact us!
Multiplex PCR assay for quantitative detection of SARS-CoV-2 and identification of SARS-CoV-2 S gene mutations
Brochure, Questions? Contact us!
Emerging SARS-CoV-2 variants
Recently, new emerging SARS-CoV-2 variants are of major public concern. These novel lineages may show increased transmissibility and even bear the risk of immune evasion, therefore posing additional global challenges in the attempt to control the viral spread as well as threatening the success of vaccination strategies.
Identifying these variants helps to track their spread and enables epidemiological surveillance.
FluoroType® SARS-CoV-2 varID Q
FluoroType® SARS-CoV-2 varID Q is a multiplex PCR test for detection and quantification of SARS-CoV-2 as well as simultaneous identification of four different S gene mutations of SARS-CoV-2.
The test is validated for use with nasopharyngeal and oropharyngeal swabs and contains reagents for up to 96 reactions. PCR results are produced in under two hours. Elaborate Internal Controls (Universal Internal Control 2, available as a separate product) monitor extraction, reverse-transcription and amplification for maximum confidence.
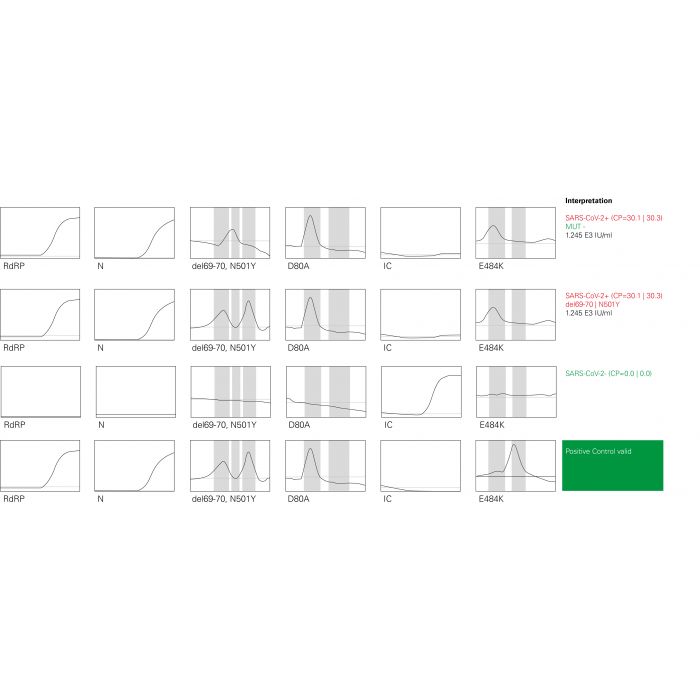
Results at a glance in combination with FluoroCycler® XT
When used in combination with the FluoroCycler® XT, the integrated FluoroSoftware® XT-IVD produces an unambiguous report that can be read ‘at a glance’. The wizard-based software comfortably guides users through setup and results evaluation. The Internal Control and software assisted evaluation ensures the generation of reliable results. Automated nucleic acid extraction for optimal performance
The GenoXtract® and GenoXtract® fleXT nucleic acid extraction systems in combination with dedicated extraction kits provide high quality nucleic acid extraction for optimal performance.
GenoXtract® allows flexible processing of 1 – 12 samples with ready-to-use reagent cartridges and consumables in a low to medium throughput setting. Users can process samples with confidence thanks to secure sample processing and unique disposable pipetting system, which minimize the potential for contamination. To support medium throughput workflows, multiple instruments can be installed in parallel. GenoXtract® fleXT is an automated extraction device for high throughput workflows with a flexible option for PCR-plate setup. Up to 96 samples can be processed directly from patient specimens.
FluoroType® SARS-CoV-2 varID Q is also validated with other extraction options. Contact us for more details.
Your benefits with FluoroType® SARS-CoV-2 varID Q
Identification of relevant SARS-CoV-2 S gene mutations: Identification of N501Y, del 69-70, D80A, E484K via melting curve analysis
Quantification of SARS-CoV-2: Viral load assessment according to the NIBSC First WHO International Standard for SARS-CoV-2 RNA (in IU/ml)
Dual target detection of SARS-CoV-2: Two independent genes of the SARS-CoV-2 genome are targeted, providing confident identification of SARS-CoV-2.
Combination with FluoroCycler® XT provides ‘results at a glance’:
Automatically generated reports allow for fast, confident reporting without the need for individual interpretation.
Validated workflow supports ease of laboratory implementation: The combination of FluoroType® SARS-CoV-2 varID Q, FluoroCycler® XT and GenoXtract® or GenoXtract® fleXT provides a fully validated workflow for ease of implementation in your laboratory.
The performance characteristics of FluoroType® SARS-CoV-2 varID Q, FluoroType® SARS-CoV-2 plus and FluoroType® SARS-CoV-2/Flu/RSV are unaffected by the mutations of the SARS-CoV-2 line age B.1.1.7 (United Kingdom), as well as the variants B.1.351 (South Africa), P.1 (Brazil) or B.1.617 (India).
Sequence comparison via in silico analysis showed no influence of the reported mutations on there liability for detection of the described SARS-CoV-2 variants by all mentioned FluoroType® assays.
Recently, new emerging SARS-CoV-2 variants are of major public concern. These novel lineages may show increased transmissibility and even bear the risk of immune evasion, therefore posing additional global challenges in the attempt to control the viral spread as well as threatening the success of vaccination strategies.
Identifying these variants helps to track their spread and enables epidemiological surveillance.
FluoroType® SARS-CoV-2 varID Q
FluoroType® SARS-CoV-2 varID Q is a multiplex PCR test for detection and quantification of SARS-CoV-2 as well as simultaneous identification of four different S gene mutations of SARS-CoV-2.
The test is validated for use with nasopharyngeal and oropharyngeal swabs and contains reagents for up to 96 reactions. PCR results are produced in under two hours. Elaborate Internal Controls (Universal Internal Control 2, available as a separate product) monitor extraction, reverse-transcription and amplification for maximum confidence.
Results at a glance in combination with FluoroCycler® XT
When used in combination with the FluoroCycler® XT, the integrated FluoroSoftware® XT-IVD produces an unambiguous report that can be read ‘at a glance’. The wizard-based software comfortably guides users through setup and results evaluation. The Internal Control and software assisted evaluation ensures the generation of reliable results. Automated nucleic acid extraction for optimal performance
The GenoXtract® and GenoXtract® fleXT nucleic acid extraction systems in combination with dedicated extraction kits provide high quality nucleic acid extraction for optimal performance.
GenoXtract® allows flexible processing of 1 – 12 samples with ready-to-use reagent cartridges and consumables in a low to medium throughput setting. Users can process samples with confidence thanks to secure sample processing and unique disposable pipetting system, which minimize the potential for contamination. To support medium throughput workflows, multiple instruments can be installed in parallel. GenoXtract® fleXT is an automated extraction device for high throughput workflows with a flexible option for PCR-plate setup. Up to 96 samples can be processed directly from patient specimens.
FluoroType® SARS-CoV-2 varID Q is also validated with other extraction options. Contact us for more details.
Your benefits with FluoroType® SARS-CoV-2 varID Q
Identification of relevant SARS-CoV-2 S gene mutations: Identification of N501Y, del 69-70, D80A, E484K via melting curve analysis
Quantification of SARS-CoV-2: Viral load assessment according to the NIBSC First WHO International Standard for SARS-CoV-2 RNA (in IU/ml)
Dual target detection of SARS-CoV-2: Two independent genes of the SARS-CoV-2 genome are targeted, providing confident identification of SARS-CoV-2.
Combination with FluoroCycler® XT provides ‘results at a glance’:
Automatically generated reports allow for fast, confident reporting without the need for individual interpretation.
Validated workflow supports ease of laboratory implementation: The combination of FluoroType® SARS-CoV-2 varID Q, FluoroCycler® XT and GenoXtract® or GenoXtract® fleXT provides a fully validated workflow for ease of implementation in your laboratory.
The performance characteristics of FluoroType® SARS-CoV-2 varID Q, FluoroType® SARS-CoV-2 plus and FluoroType® SARS-CoV-2/Flu/RSV are unaffected by the mutations of the SARS-CoV-2 line age B.1.1.7 (United Kingdom), as well as the variants B.1.351 (South Africa), P.1 (Brazil) or B.1.617 (India).
Sequence comparison via in silico analysis showed no influence of the reported mutations on there liability for detection of the described SARS-CoV-2 variants by all mentioned FluoroType® assays.
| Is Featured? | No |
|---|
Write Your Own Review