GenoType CVD Thrombophilia PCR CE/IVD
€0.00
In stock
SKU
HL-33512
Catalog Number: HL-33512, Size: 12 tests
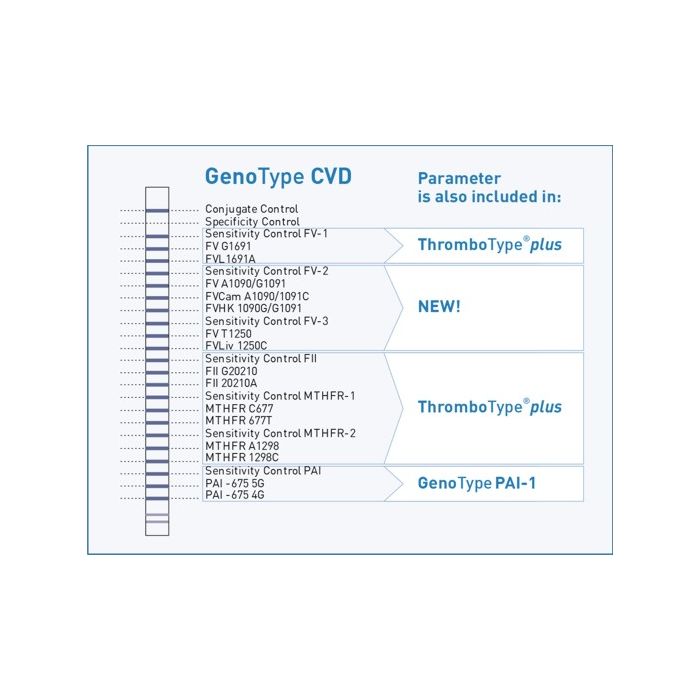
Molecular genetic assay to detect following thrombophilia associated mutations: Factor V Leiden, Cambridge, Hong Kong, Liverpool, Prothrombin G20210A, MTHFR C677T, MTHFR A1298C and PAI -675 4G/5G
Flyer, Questions? Contact us!
Molecular genetic assay to detect following thrombophilia associated mutations: Factor V Leiden, Cambridge, Hong Kong, Liverpool, Prothrombin G20210A, MTHFR C677T, MTHFR A1298C and PAI -675 4G/5G
Flyer, Questions? Contact us!
It is estimated that 2 of 1,000 people worldwide are affected by thrombophilia. The individual risk of disease depends on different factors. Genetically caused blood-clotting disorders play a major role. The Factor V Leiden mutation is the most common thrombophilia-associated mutation. A point mutation within the Factor V gene causes an amino acid exchange of arginine versus glutamine. The mutation eliminates the activated protein C (APC) cleavage site, thus the result is an increased stimulation of blood coagulation. The second most frequent mutation is the prothrombin G20210A mutation. This point mutation involves the noncoding regulatory area of the Factor II gene and leads to an increased prothrombin concentration in plasma.
Most patients who suffer from thrombophilia do have more than one genetic predisposition. A combination of genetic defects leads to an increased risk for thrombosis, thus a combined analysis of thrombophilia-associated parameters is reasonable. Mutations within the Factor V gene as Factor V Cambridge, Factor V Hong Kong and Factor V Liverpool are rare but still relevant for thrombophilia and should not be disregarded. The identification of these parameters in combination with further thrombophilia-associated mutations as MTHFR C677T, MTHFR A1298C and PAI -675 4G/5G permits a reliable estimation of the individual risk for thrombosis.
GenoType CVD enables the detection of these eight different thrombophilia-associated mutations for broad and reliable thrombophilia diagnostics.
Your benefits with GenoType CVD
- User-friendly: The test system is based on the user-friendly DNA•STRIP technology. A ready-to-use amplification mix is already included and also the Taq polymerase is provided in the kit. This saves time and money enabling an optimal integration in your daily routine.
- Individual automation: DNA isolation and detection can be performed automatically. Batch or individual automation can be performed, thus gaining maximum flexibility.
- Simple evaluation: using an evaluation template, the test result can be read rapidly and clearly.
Molecular genetic assay to detect following thrombophilia associated mutations: Factor V Leiden, Cambridge, Hong Kong, Liverpool, Prothrombin G20210A, MTHFR C677T, MTHFR A1298C and PAI -675 4G/5G
Starting material:
EDTA/Citrate blood
DNA Isolation:
GenoType DNA Isolation Kit or
GXT Blood Extraction Kit (with GenoXtract®)
Most patients who suffer from thrombophilia do have more than one genetic predisposition. A combination of genetic defects leads to an increased risk for thrombosis, thus a combined analysis of thrombophilia-associated parameters is reasonable. Mutations within the Factor V gene as Factor V Cambridge, Factor V Hong Kong and Factor V Liverpool are rare but still relevant for thrombophilia and should not be disregarded. The identification of these parameters in combination with further thrombophilia-associated mutations as MTHFR C677T, MTHFR A1298C and PAI -675 4G/5G permits a reliable estimation of the individual risk for thrombosis.
GenoType CVD enables the detection of these eight different thrombophilia-associated mutations for broad and reliable thrombophilia diagnostics.
Your benefits with GenoType CVD
- User-friendly: The test system is based on the user-friendly DNA•STRIP technology. A ready-to-use amplification mix is already included and also the Taq polymerase is provided in the kit. This saves time and money enabling an optimal integration in your daily routine.
- Individual automation: DNA isolation and detection can be performed automatically. Batch or individual automation can be performed, thus gaining maximum flexibility.
- Simple evaluation: using an evaluation template, the test result can be read rapidly and clearly.
Molecular genetic assay to detect following thrombophilia associated mutations: Factor V Leiden, Cambridge, Hong Kong, Liverpool, Prothrombin G20210A, MTHFR C677T, MTHFR A1298C and PAI -675 4G/5G
Starting material:
EDTA/Citrate blood
DNA Isolation:
GenoType DNA Isolation Kit or
GXT Blood Extraction Kit (with GenoXtract®)
| Is Featured? | No |
|---|
Write Your Own Review