HeMoStep - Detect/Quantify blood contamination in cerebrospinal fluid CE-IVD
€0.00
In stock
SKU
IS-IMS1510
Catalog Number: IS-IMS1510
Size: 50 test/kit
Diagnostic kit designed to quantify platelet associated immunoglobulin using Flow Cytometry
Datasheet
Flyer
Poster
Questions? Contact us!
Size: 50 test/kit
Diagnostic kit designed to quantify platelet associated immunoglobulin using Flow Cytometry
Datasheet
Flyer
Poster
Questions? Contact us!
High sensitivity, accuracy, and reliability in just 55 minutes.
Welcome to the future of CSF testing! We are thrilled to introduce our groundbreaking method that will revolutionize how you DETECT and QUANTIFY blood contamination in cerebrospinal fluid samples.
With relative frequency, lumbar puncture procedure is complicated by the contamination of CSF with blood cells as result of needle trauma, also known as traumatic tap.
No more confusing diagnoses and analysis complications due to the presence of blood in Cerebrospinal Fluid samples thanks to the HemoStep Kit.
INTENDED USE
The HeMoStep kit is an in vitro immunoassay for the quantitative measurement of blood contamination of cerebrospinal fluid (CSF) samples by flow cytometry.
The intended use of the assay is to quantify the contamination of CSF samples with peripheral blood, helping, in combination with other tests such as flow cytometry (FCM) and cytology (CC), to the accurate interpretation of the results from the analysis of this type of samples (CSF) improving the diagnosis of leptomeningeal disease in patients with B and T cell lymphomas and acute leukemias of lymphoid and myeloid origin
CSF fluid cell analysis is an important clinical procedure for the diagnosis, classification and prognosis of a wide variety of diseases. CSF sampling is performed by a procedure called lumbar puncture (LP), which involves inserting a needle into the spinal canal. During the procedure, the needle passes through several layers of vascularised tissue until it reaches the subarachnoid space and peripheral blood (PB) may be introduced into the sample tube contaminating the CSF. In addition, the puncture can sometimes be complicated by increased bleeding, called traumatic LP (up to 20%), resulting in visible red contamination of the CSF sample. This type of puncture is called traumatic 1. Thus, the presence of blood cells and the affected concentration of some substances due to blood contamination in the CSF can complicate the analysis and confuse the diagnosis.
Multiparametric FCM immunophenotyping combines high specificity with good clinical sensitivity and several studies and guidelines recommend this immunophenotyping for efficient and reliable CSF diagnosis in patients with haematological malignancies such as B- and T-cell lymphomas and acute leukaemias of lymphoid and myeloid origin, in whom CSF tumour infiltration is suspected.
Assessment of CSF contamination with SP should be performed in all cases and especially in cases where the presence of blood cells is observed and when malignant cells are present in peripheral blood. In this sense, although the cerebrospinal fluid is crystalline, it is common for it to present an alteration in colour due either to pathological bleeding in the central nervous system (CNS) or, as we have already mentioned, to a traumatic puncture. In the latter case, it is difficult to evaluate the degree of contamination by visual inspection alone, as the visual threshold for the perception of blood in cerebrospinal fluid varies from 400 to 6000 red blood cells (RBCs) per mm3, according to different authors1,5-6 and even visibly undetectable blood contamination can drastically alter the CSF content.
Some of the most commonly used methods for estimation of CSF contamination by CMF are based on immunophenotyping and enumeration of absolute contaminating cells from peripheral blood, mainly RBCs and/or neutrophils. However, such protocols have limitations (low precision and low sensitivity) related to cell loss during the concentration/centrifugation steps, but also to cell destruction due to the rapid in vitro cytotoxic effects of CSF on leukocytes and thus on neutrophils.
The assessment of CSF sample quality is essential in the diagnosis and follow-up of leukaemias and lymphomas and having a method that circumvents the disadvantages associated with the cell destruction of this type of analysis can be a determining factor in obtaining good results.
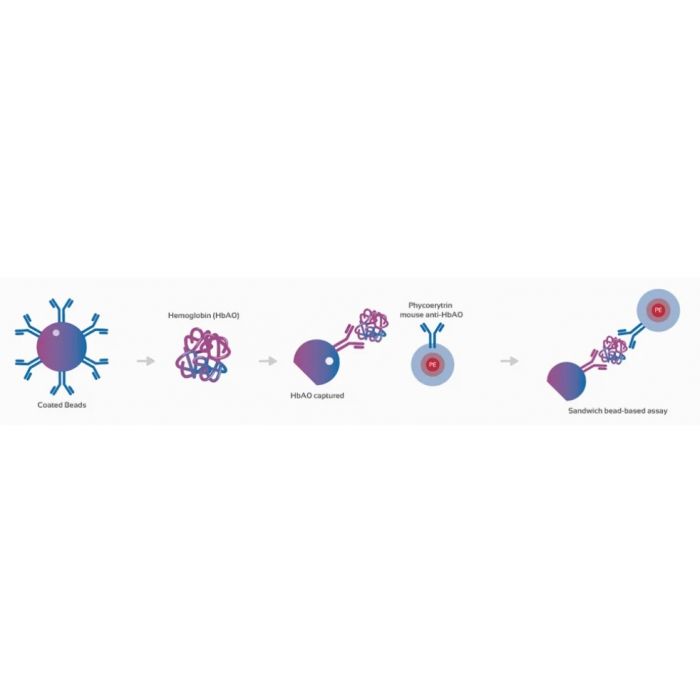
This kit, based on the quantification of total haemoglobin (Hb), a specific biomarker for RBCs, helps to interpret diagnostic results, as it circumvents the problems arising from CSF cytotoxicity, minimizes sample usage, which is particularly important in paucicellular samples and significantly improves sensitivity for the determination of CSF contamination with PB.
Kit contents
Capture microspheres coated with an Hb-specific monoclonal antibody.
Magnetic polystyrene microspheres (mean diameter 6 μm), are supplied in 2 vials at the following concentration: 2000 microspheres/test (50μl/test) - 2,5ml/vial and in a buffered aqueous solution containing protein stabilizer and 0.09% sodium azide (NaN3), as an anti-microbial agent.
25 ml wash buffer (10X)
PBS 10% BSA, pH 7.4 - 10X. Contains 10% albumin in 10mM sodium phosphate, 150mM NaCl, pH 7.4, contains KATHON™ anti-microbial agent.
Dilute the contents of the 10X assay buffer to 1X (PBS 1% BSA) in PBS, pH 7.4, for use in this assay.
Positive control. 5 vials of lyophilized RBCs lysate.
Solubility H2O. Reconstitute before use
Calibration microspheres capped with two different concentrations of Hb
2 different populations of magnetic polystyrene microspheres (mean diameter 6 μm) and an internal fluorescence standard different from the capture microspheres are supplied in 1 vial at the following concentration: 2000 (1000 beads of each) microspheres/test (50μl/test) - 1ml/vial and in a buffered aqueous solution containing protein stabilizer and 0.09% sodium azide (NaN3) as an anti-microbial agent
Standard of known concentration. 1 vial of lyophilized RBCs lysate with known concentrations of [Hb].
Solubility H2O. Reconstitute before use.
To be used to generate the standard curve.
1.2 ml fluorescently conjugated (PE) detector antibody (10 μl/test).
The antibody is supplied in 2 vials (0.6 ml/vial), use concentration and buffered aqueous solution containing protein stabilizer and 0.09% sodium azide (NaN3) as an antimicrobial agent.
Welcome to the future of CSF testing! We are thrilled to introduce our groundbreaking method that will revolutionize how you DETECT and QUANTIFY blood contamination in cerebrospinal fluid samples.
With relative frequency, lumbar puncture procedure is complicated by the contamination of CSF with blood cells as result of needle trauma, also known as traumatic tap.
No more confusing diagnoses and analysis complications due to the presence of blood in Cerebrospinal Fluid samples thanks to the HemoStep Kit.
INTENDED USE
The HeMoStep kit is an in vitro immunoassay for the quantitative measurement of blood contamination of cerebrospinal fluid (CSF) samples by flow cytometry.
The intended use of the assay is to quantify the contamination of CSF samples with peripheral blood, helping, in combination with other tests such as flow cytometry (FCM) and cytology (CC), to the accurate interpretation of the results from the analysis of this type of samples (CSF) improving the diagnosis of leptomeningeal disease in patients with B and T cell lymphomas and acute leukemias of lymphoid and myeloid origin
CSF fluid cell analysis is an important clinical procedure for the diagnosis, classification and prognosis of a wide variety of diseases. CSF sampling is performed by a procedure called lumbar puncture (LP), which involves inserting a needle into the spinal canal. During the procedure, the needle passes through several layers of vascularised tissue until it reaches the subarachnoid space and peripheral blood (PB) may be introduced into the sample tube contaminating the CSF. In addition, the puncture can sometimes be complicated by increased bleeding, called traumatic LP (up to 20%), resulting in visible red contamination of the CSF sample. This type of puncture is called traumatic 1. Thus, the presence of blood cells and the affected concentration of some substances due to blood contamination in the CSF can complicate the analysis and confuse the diagnosis.
Multiparametric FCM immunophenotyping combines high specificity with good clinical sensitivity and several studies and guidelines recommend this immunophenotyping for efficient and reliable CSF diagnosis in patients with haematological malignancies such as B- and T-cell lymphomas and acute leukaemias of lymphoid and myeloid origin, in whom CSF tumour infiltration is suspected.
Assessment of CSF contamination with SP should be performed in all cases and especially in cases where the presence of blood cells is observed and when malignant cells are present in peripheral blood. In this sense, although the cerebrospinal fluid is crystalline, it is common for it to present an alteration in colour due either to pathological bleeding in the central nervous system (CNS) or, as we have already mentioned, to a traumatic puncture. In the latter case, it is difficult to evaluate the degree of contamination by visual inspection alone, as the visual threshold for the perception of blood in cerebrospinal fluid varies from 400 to 6000 red blood cells (RBCs) per mm3, according to different authors1,5-6 and even visibly undetectable blood contamination can drastically alter the CSF content.
Some of the most commonly used methods for estimation of CSF contamination by CMF are based on immunophenotyping and enumeration of absolute contaminating cells from peripheral blood, mainly RBCs and/or neutrophils. However, such protocols have limitations (low precision and low sensitivity) related to cell loss during the concentration/centrifugation steps, but also to cell destruction due to the rapid in vitro cytotoxic effects of CSF on leukocytes and thus on neutrophils.
The assessment of CSF sample quality is essential in the diagnosis and follow-up of leukaemias and lymphomas and having a method that circumvents the disadvantages associated with the cell destruction of this type of analysis can be a determining factor in obtaining good results.
This kit, based on the quantification of total haemoglobin (Hb), a specific biomarker for RBCs, helps to interpret diagnostic results, as it circumvents the problems arising from CSF cytotoxicity, minimizes sample usage, which is particularly important in paucicellular samples and significantly improves sensitivity for the determination of CSF contamination with PB.
Kit contents
Capture microspheres coated with an Hb-specific monoclonal antibody.
Magnetic polystyrene microspheres (mean diameter 6 μm), are supplied in 2 vials at the following concentration: 2000 microspheres/test (50μl/test) - 2,5ml/vial and in a buffered aqueous solution containing protein stabilizer and 0.09% sodium azide (NaN3), as an anti-microbial agent.
25 ml wash buffer (10X)
PBS 10% BSA, pH 7.4 - 10X. Contains 10% albumin in 10mM sodium phosphate, 150mM NaCl, pH 7.4, contains KATHON™ anti-microbial agent.
Dilute the contents of the 10X assay buffer to 1X (PBS 1% BSA) in PBS, pH 7.4, for use in this assay.
Positive control. 5 vials of lyophilized RBCs lysate.
Solubility H2O. Reconstitute before use
Calibration microspheres capped with two different concentrations of Hb
2 different populations of magnetic polystyrene microspheres (mean diameter 6 μm) and an internal fluorescence standard different from the capture microspheres are supplied in 1 vial at the following concentration: 2000 (1000 beads of each) microspheres/test (50μl/test) - 1ml/vial and in a buffered aqueous solution containing protein stabilizer and 0.09% sodium azide (NaN3) as an anti-microbial agent
Standard of known concentration. 1 vial of lyophilized RBCs lysate with known concentrations of [Hb].
Solubility H2O. Reconstitute before use.
To be used to generate the standard curve.
1.2 ml fluorescently conjugated (PE) detector antibody (10 μl/test).
The antibody is supplied in 2 vials (0.6 ml/vial), use concentration and buffered aqueous solution containing protein stabilizer and 0.09% sodium azide (NaN3) as an antimicrobial agent.
| Is Featured? | No |
|---|
Write Your Own Review