Metal-Chelating Separopore® 4B-CL
€0.00
In stock
SKU
BW-20181020
Application:
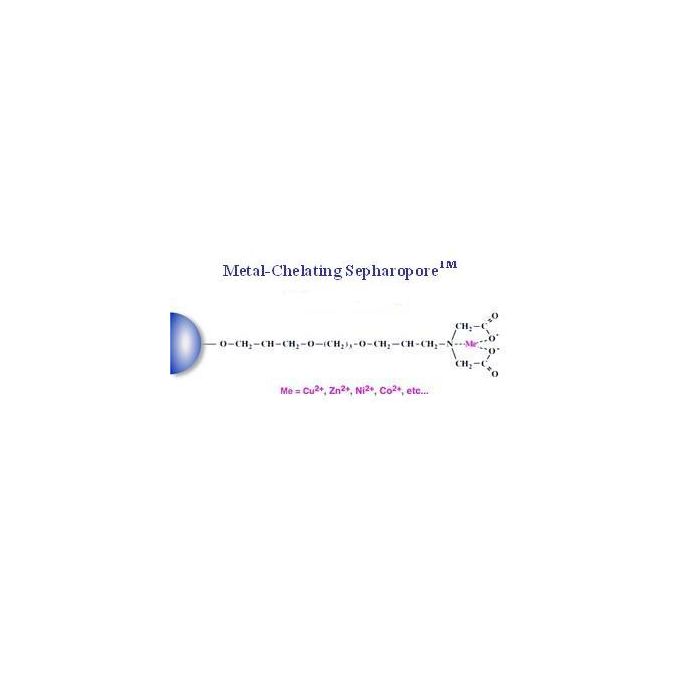
Metal ion-chelating (IDA) Separopore® 4B-CL is designed for the affinity purification of 6X Histidine-tagged proteins. Nickel, Cobalt, Copper and Zinc are used as cations for this type of purification because they provide effective binding and selectivity for a wide range of proteins. Affinity matrix for proteins with metal binding properties with exposed amino acids such as histidine and cysteine.
Metal ion-chelating Separopore® 4B-CL can be charged with Zn2+, Cu2+, Co2+ and Ni2+ metal ions. Imminodiacetic acid immobilized on the matrix allows versatile chelation of divalent metal ions of choice. This is ideal for purification of recombinant proteins containing (His)6 fusion tag and small-scale affinity capture (molecular pull-down) purifications of histidine-tagged protein while exhibiting low non-specific binding of other proteins. Metal ion-chelating Separopore® 4B-CL contain a quadridentate chelate, which is bound with Zn2+, Cu2+, Co2+ and Ni2+ metal ions and covalently attached through a non-charged, hydrophilic linker to Separopore®. Low metal ion leakage means that the activity of the purified protein is retained and the risk of precipitation reduced, which results in increased purity, activity, and yield of the target protein. Leakage of metal ions into the eluted protein pool from beads is generally low under normal conditions.
Note: Separopore® is a cost-effective equivalent to Sepharose® in all of its physical properties and binding characteristics.
Technical Specifications:
Chelating group: None (Imminodiacetic acid (IDA) activated)
Matrix: Separopore® 4B-CL (crosslinked agarose beads, 4%)
Particle size range: 52 – 165 µm
Chelating group coupling method: Epoxy activation
Spacer arm: 14 atoms
Metal ion capacity: >20 µmol Me2+ /ml drained gel
Binding capacity: >50 mg (His)6-tagged protein / ml drained gel
pH stability: 3 – 12
Storage: 4 – 30 °C
Supplied as suspension in 20% ethanol
References:
Zinc-binding proteins from boar seminal plasma -- isolation, biochemical characteristics and influence on spermatozoa stored at 4°C. Acta Biochim Pol. (2011) 58: 171-7.
Metal dependent hydrolysis of β-casein by sIgA antibodies from human milk. J Mol Recognit. (2011) 24: 45-59.
Determination of trace metal ions via on-line separation and preconcentration by means of chelating Sepharose beads in a sequential injection lab-on-valve (SI-LOV) system coupled to electrothermal atomic absorption spectrometric detection. Talanta. (2005) 66: 1326-32.
High-level expression of his-tagged clostridial collagenase in Clostridium perfringens. Appl Microbiol Biotechnol. (2008) 80: 627-35.
Efficient preparation of an acyclic permutant of kalata B1 from a recombinant fusion protein with thioredoxin. J Biotechnol. (2007) 130: 378-84.
Recombinant sucrose phosphorylase from Leuconostoc mesenteroides: characterization, kinetic studies of transglucosylation, and application of immobilised enzyme for production of alpha-D-glucose 1-phosphate. J Biotechnol. (2007) 129: 77-86.
Water-elutability of nucleic acids from metal-chelate affinity adsorbents: enhancement by control of surface charge density. J Mol Recognit. (2006) 19: 348-53.
Metal-dependent hydrolysis of myelin basic protein by IgGs from the sera of patients with multiple sclerosis. Immunol Lett. (2006) 103: 75-81.
Porcine, mouse and human galactose 3-O-sulphotransferase-2 enzymes have different substrate specificities; the porcine enzyme requires basic compounds for its catalytic activity. Biochem J. (2005) 391: 77-85.
Activity assay of His-tagged E. coli DNA photolyase by RP-HPLC and SE-HPLC. J Biochem Biophys Methods. (2005) 63: 111-24.
Purification of native dehydrin from Glycine Max cv., Pisum sativum, and Rosmarinum officinalis by affinity chromatography. Protein Expr Purif. (2003) 28: 232-40.
Calorimetric analysis of cephalosporins using an immobilized TEM-1 beta-lactamase on Ni2+ chelating sepharose fast flow. Anal Biochem. (2001) 296: 57-62.
One-step chromatographic purification procedure of a His-tag recombinant carboxyl half part of the HTLV-I surface envelope glycoprotein overexpressed in Escherichia coli as a secreted form. J Chromatogr B Biomed Sci Appl. (2001) 753: 17-22.
Cloning, expression, purification, and characterization of rat MMP-12. Protein Expr Purif. (2001) 21: 268-74.
A modified metal-ion affinity chromatography procedure for the purification of histidine-tagged recombinant proteins expressed in Drosophila S2 cells. Protein Expr Purif. (2000) 19: 362-8.
Covalent and metal-chelate immobilization of a modified 2-haloacid dehalogenase for the enzymatic resolution of optically active chloropropionic acid. Biotechnol Prog. (2000) 16: 287-91.
Immobilised metal ion affinity chromatography purification of alcohol dehydrogenase from baker's yeast using an expanded bed adsorption system. J Chromatogr A. (1999) 840: 195-204.
State of imidazole side chain of hen lysozyme modified with histamine and Japanese quail lysozyme. A study by immobilized metal ion affinity chromatography. Biosci Biotechnol Biochem. (1998) 62: 2239-41.
Recombinant human amyloid precursor-like protein 2 (APLP2) expressed in the yeast Pichia pastoris can stimulate neurite outgrowth. FEBS Lett. (1999) 442: 95-8.
Related products:
Co2+-chelated Separopore® 4B-CL (SKU # 20181047)
Cu2+-chelated Separopore® 4B-CL (SKU # 20181048)
Ni2+-chelated Separopore® 4B-CL (SKU # 20181046)
Zn2+-Chelated Separopore® 4B-CL (SKU # 20181049)
Usage: bioWORLD's products are supplied for LABORATORY RESEARCH USE ONLY. The product may not be used as a drug, agricultural or pesticidal product , food additive or as a household chemical.
Hazmat Shipping: Non-hazardous
Storage: 2-8°C
Brand Name: bioPLUS™, Separopore®
Metal ion-chelating (IDA) Separopore® 4B-CL is designed for the affinity purification of 6X Histidine-tagged proteins. Nickel, Cobalt, Copper and Zinc are used as cations for this type of purification because they provide effective binding and selectivity for a wide range of proteins. Affinity matrix for proteins with metal binding properties with exposed amino acids such as histidine and cysteine.
Metal ion-chelating Separopore® 4B-CL can be charged with Zn2+, Cu2+, Co2+ and Ni2+ metal ions. Imminodiacetic acid immobilized on the matrix allows versatile chelation of divalent metal ions of choice. This is ideal for purification of recombinant proteins containing (His)6 fusion tag and small-scale affinity capture (molecular pull-down) purifications of histidine-tagged protein while exhibiting low non-specific binding of other proteins. Metal ion-chelating Separopore® 4B-CL contain a quadridentate chelate, which is bound with Zn2+, Cu2+, Co2+ and Ni2+ metal ions and covalently attached through a non-charged, hydrophilic linker to Separopore®. Low metal ion leakage means that the activity of the purified protein is retained and the risk of precipitation reduced, which results in increased purity, activity, and yield of the target protein. Leakage of metal ions into the eluted protein pool from beads is generally low under normal conditions.
Note: Separopore® is a cost-effective equivalent to Sepharose® in all of its physical properties and binding characteristics.
Technical Specifications:
Chelating group: None (Imminodiacetic acid (IDA) activated)
Matrix: Separopore® 4B-CL (crosslinked agarose beads, 4%)
Particle size range: 52 – 165 µm
Chelating group coupling method: Epoxy activation
Spacer arm: 14 atoms
Metal ion capacity: >20 µmol Me2+ /ml drained gel
Binding capacity: >50 mg (His)6-tagged protein / ml drained gel
pH stability: 3 – 12
Storage: 4 – 30 °C
Supplied as suspension in 20% ethanol
References:
Zinc-binding proteins from boar seminal plasma -- isolation, biochemical characteristics and influence on spermatozoa stored at 4°C. Acta Biochim Pol. (2011) 58: 171-7.
Metal dependent hydrolysis of β-casein by sIgA antibodies from human milk. J Mol Recognit. (2011) 24: 45-59.
Determination of trace metal ions via on-line separation and preconcentration by means of chelating Sepharose beads in a sequential injection lab-on-valve (SI-LOV) system coupled to electrothermal atomic absorption spectrometric detection. Talanta. (2005) 66: 1326-32.
High-level expression of his-tagged clostridial collagenase in Clostridium perfringens. Appl Microbiol Biotechnol. (2008) 80: 627-35.
Efficient preparation of an acyclic permutant of kalata B1 from a recombinant fusion protein with thioredoxin. J Biotechnol. (2007) 130: 378-84.
Recombinant sucrose phosphorylase from Leuconostoc mesenteroides: characterization, kinetic studies of transglucosylation, and application of immobilised enzyme for production of alpha-D-glucose 1-phosphate. J Biotechnol. (2007) 129: 77-86.
Water-elutability of nucleic acids from metal-chelate affinity adsorbents: enhancement by control of surface charge density. J Mol Recognit. (2006) 19: 348-53.
Metal-dependent hydrolysis of myelin basic protein by IgGs from the sera of patients with multiple sclerosis. Immunol Lett. (2006) 103: 75-81.
Porcine, mouse and human galactose 3-O-sulphotransferase-2 enzymes have different substrate specificities; the porcine enzyme requires basic compounds for its catalytic activity. Biochem J. (2005) 391: 77-85.
Activity assay of His-tagged E. coli DNA photolyase by RP-HPLC and SE-HPLC. J Biochem Biophys Methods. (2005) 63: 111-24.
Purification of native dehydrin from Glycine Max cv., Pisum sativum, and Rosmarinum officinalis by affinity chromatography. Protein Expr Purif. (2003) 28: 232-40.
Calorimetric analysis of cephalosporins using an immobilized TEM-1 beta-lactamase on Ni2+ chelating sepharose fast flow. Anal Biochem. (2001) 296: 57-62.
One-step chromatographic purification procedure of a His-tag recombinant carboxyl half part of the HTLV-I surface envelope glycoprotein overexpressed in Escherichia coli as a secreted form. J Chromatogr B Biomed Sci Appl. (2001) 753: 17-22.
Cloning, expression, purification, and characterization of rat MMP-12. Protein Expr Purif. (2001) 21: 268-74.
A modified metal-ion affinity chromatography procedure for the purification of histidine-tagged recombinant proteins expressed in Drosophila S2 cells. Protein Expr Purif. (2000) 19: 362-8.
Covalent and metal-chelate immobilization of a modified 2-haloacid dehalogenase for the enzymatic resolution of optically active chloropropionic acid. Biotechnol Prog. (2000) 16: 287-91.
Immobilised metal ion affinity chromatography purification of alcohol dehydrogenase from baker's yeast using an expanded bed adsorption system. J Chromatogr A. (1999) 840: 195-204.
State of imidazole side chain of hen lysozyme modified with histamine and Japanese quail lysozyme. A study by immobilized metal ion affinity chromatography. Biosci Biotechnol Biochem. (1998) 62: 2239-41.
Recombinant human amyloid precursor-like protein 2 (APLP2) expressed in the yeast Pichia pastoris can stimulate neurite outgrowth. FEBS Lett. (1999) 442: 95-8.
Related products:
Co2+-chelated Separopore® 4B-CL (SKU # 20181047)
Cu2+-chelated Separopore® 4B-CL (SKU # 20181048)
Ni2+-chelated Separopore® 4B-CL (SKU # 20181046)
Zn2+-Chelated Separopore® 4B-CL (SKU # 20181049)
Usage: bioWORLD's products are supplied for LABORATORY RESEARCH USE ONLY. The product may not be used as a drug, agricultural or pesticidal product , food additive or as a household chemical.
Hazmat Shipping: Non-hazardous
Storage: 2-8°C
Brand Name: bioPLUS™, Separopore®
| Is Featured? | No |
|---|
Write Your Own Review