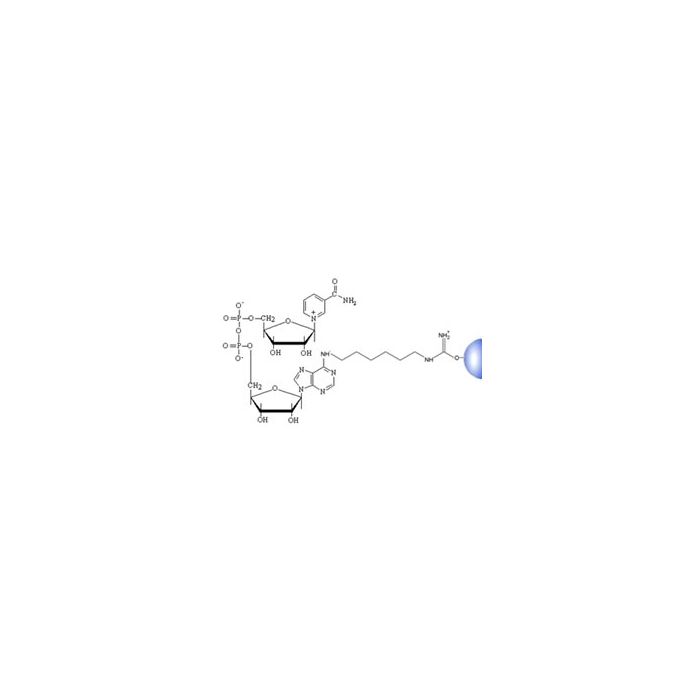
NAD Separopore® 4B-CL
€0.00
In stock
SKU
BW-20181024
Application:
NAD covalently bound to Separopore® has a high affinity for NAD-dependent enzymes and dehydrogenases. NAD-Separopore® 4B-CL has been used to purify Vibrio cholerae toxin, diptheria toxin and other NAD binding proteins.
Note: Separopore® is a cost-effective equivalent to Sepharose® in all of its physical properties and binding characteristics.
Technical Specifications:
Ligand: NAD
Matrix: Separopore® 4B-CL (crosslinked agarose beads, 4%)
Particle size range: 52 – 165 µm
Matrix activation: Cyanogen bromide
Matrix attachment: N-6
Ligand density: 2 µmol NAD / ml drained gel
pH stability: 4 – 10
Storage: -20 °C
Supplied as lyophilized powder stabilized with lactose
Swelling: 1 g swells to approximately 5 ml
References:
Purification of the poly-ADP-ribose polymerase-like thermozyme from the archaeon Sulfolobus solfataricus. Methods Mol Biol. (2011) 780: 443-60.
Allosteric behaviour of 1:5 hybrids of mutant subunits of Clostridium symbiosum glutamate dehydrogenase differing in their amino acid specificity. Biochem J. (2001) 360: 651-6.
The staphylococcal transferrin-binding protein is a cell wall glyceraldehyde-3-phosphate dehydrogenase. Infect Immun. (1999) 67: 1086-92.
Purification and biochemical characterization of a poly(ADP-ribose) polymerase-like enzyme from the thermophilic archaeon Sulfolobus solfataricus. Biochem J. (1998) 335: 441-7.
Use of protein engineering to explore subunit interactions in an allosteric enzyme: construction of inter-subunit hybrids in Clostridium symbiosum glutamate dehydrogenase. Protein Eng. (1998) 11: 569-75.
Purification of NAD-dependent mannitol dehydrogenase from celery suspension cultures. Plant Physiol. (1995) 108: 1219-25.
Evidence for the presence of two pyridine nucleotide-binding sites on the beta subunit of the Escherichia coli pyridine nucleotide transhydrogenase. Biochem Mol Biol Int. (1995) 35: 297-306.
Purification and partial characterization of microsomal NADH-cytochrome b5 reductase from higher plant Catharanthus roseus. Biochem Biophys Res Commun. (1993) 197: 518-22.
A mutation at Gly314 of the beta subunit of the Escherichia coli pyridine nucleotide transhydrogenase abolishes activity and affects the NADP(H)-induced conformational change. Eur J Biochem. (1992) 207: 733-9.
Mechanistic studies of the biosynthesis of 3,6-dideoxyhexoses in Yersinia pseudotuberculosis. Purification and stereochemical analysis of CDP-D-glucose oxidoreductase. J Biol Chem. (1992) 267: 5868-75.
Topological analysis of the pyridine nucleotide transhydrogenase of Escherichia coli using proteolytic enzymes. Biochim Biophys Acta. (1991) 1080: 19-28.
Purification and characterization of NAD(+)-dependent 15-hydroxyprostaglandin dehydrogenase from porcine kidney. Biochim Biophys Acta. (1990) 1035: 190-6.
Glyceraldehyde-3-phosphate dehydrogenase is a major protein associated with the plasma membrane of retinal photoreceptor outer segments. J Biol Chem. (1990) 265: 13308-13.
Human aldehyde dehydrogenase. Purification and characterization of a third isozyme with low Km for gamma-aminobutyraldehyde. J Biol Chem. (1989) 264: 4715-21.
A menadione-stimulated pyridine nucleotide oxidase from resting bovine neutrophil membranes. Purification, properties, and immunochemical cross-reactivity with the human neutrophil NADPH oxidase. J Biol Chem. (1988) 263: 11657-63.
Purification and characterization of two types of NADH-quinone reductase from Thermus thermophilus HB-8. Biochemistry. (1988) 27: 2008-13.
Amino acid specific ADP-ribosylation: specific NAD: arginine mono-ADP-ribosyltransferases associated with turkey erythrocyte nuclei and plasma membranes. Biochemistry. (1986) 25: 8057-62.
Purification and characterization of NADH dehydrogenase complex from Paracoccus denitrificans. Arch Biochem Biophys. (1986) 250: 302-11.
Mitochondrial nicotinamide nucleotide transhydrogenase: nonidentical modification by N,N'-dicyclohexylcarbodiimide and N-(ethoxycarbonyl)-2-ethoxy-1,2-dihydroquinoline at the NAD(H) binding site. Arch Biochem Biophys. (1985) 243: 298-304.
Purification and characterization of the secondary alcohol dehydrogenase from propane-utilizing Mycobacterium vaccae strain JOB-5. J Gen Microbiol. (1985) 131: 2901-7.
Related products:
ADP-Separopore® 4B-CL (Lyophilized) (SKU # 20181057)
AMP-Separopore® 4B-CL (Lyophilized) (SKU # 20181000)
ATP-Separopore® 4B-CL (Lyophilized) (SKU # 20181080)
ATP-Separopore® 4B-CL (SKU # 20181051)
N-Acetyl-galactosamine Separopore® 4B-CL (SKU # 20181021)
N-Acetyl-glucosamine Separopore® 4B-CL (SKU # 20181022)
GTP-Separopore® 4B-CL (SKU # 20181066)
NADP-Separopore® 4B-CL (Lyophilized) (SKU # 20181023)
Usage: bioWORLD's products are supplied for LABORATORY RESEARCH USE ONLY. The product may not be used as a drug, agricultural or pesticidal product , food additive or as a household chemical.
Hazmat Shipping: Non-hazardous
Storage: -20°C
Appearance Form: Powder
Appearance Color: Off-white
Brand Name: bioPLUS™, Separopore®
NAD covalently bound to Separopore® has a high affinity for NAD-dependent enzymes and dehydrogenases. NAD-Separopore® 4B-CL has been used to purify Vibrio cholerae toxin, diptheria toxin and other NAD binding proteins.
Note: Separopore® is a cost-effective equivalent to Sepharose® in all of its physical properties and binding characteristics.
Technical Specifications:
Ligand: NAD
Matrix: Separopore® 4B-CL (crosslinked agarose beads, 4%)
Particle size range: 52 – 165 µm
Matrix activation: Cyanogen bromide
Matrix attachment: N-6
Ligand density: 2 µmol NAD / ml drained gel
pH stability: 4 – 10
Storage: -20 °C
Supplied as lyophilized powder stabilized with lactose
Swelling: 1 g swells to approximately 5 ml
References:
Purification of the poly-ADP-ribose polymerase-like thermozyme from the archaeon Sulfolobus solfataricus. Methods Mol Biol. (2011) 780: 443-60.
Allosteric behaviour of 1:5 hybrids of mutant subunits of Clostridium symbiosum glutamate dehydrogenase differing in their amino acid specificity. Biochem J. (2001) 360: 651-6.
The staphylococcal transferrin-binding protein is a cell wall glyceraldehyde-3-phosphate dehydrogenase. Infect Immun. (1999) 67: 1086-92.
Purification and biochemical characterization of a poly(ADP-ribose) polymerase-like enzyme from the thermophilic archaeon Sulfolobus solfataricus. Biochem J. (1998) 335: 441-7.
Use of protein engineering to explore subunit interactions in an allosteric enzyme: construction of inter-subunit hybrids in Clostridium symbiosum glutamate dehydrogenase. Protein Eng. (1998) 11: 569-75.
Purification of NAD-dependent mannitol dehydrogenase from celery suspension cultures. Plant Physiol. (1995) 108: 1219-25.
Evidence for the presence of two pyridine nucleotide-binding sites on the beta subunit of the Escherichia coli pyridine nucleotide transhydrogenase. Biochem Mol Biol Int. (1995) 35: 297-306.
Purification and partial characterization of microsomal NADH-cytochrome b5 reductase from higher plant Catharanthus roseus. Biochem Biophys Res Commun. (1993) 197: 518-22.
A mutation at Gly314 of the beta subunit of the Escherichia coli pyridine nucleotide transhydrogenase abolishes activity and affects the NADP(H)-induced conformational change. Eur J Biochem. (1992) 207: 733-9.
Mechanistic studies of the biosynthesis of 3,6-dideoxyhexoses in Yersinia pseudotuberculosis. Purification and stereochemical analysis of CDP-D-glucose oxidoreductase. J Biol Chem. (1992) 267: 5868-75.
Topological analysis of the pyridine nucleotide transhydrogenase of Escherichia coli using proteolytic enzymes. Biochim Biophys Acta. (1991) 1080: 19-28.
Purification and characterization of NAD(+)-dependent 15-hydroxyprostaglandin dehydrogenase from porcine kidney. Biochim Biophys Acta. (1990) 1035: 190-6.
Glyceraldehyde-3-phosphate dehydrogenase is a major protein associated with the plasma membrane of retinal photoreceptor outer segments. J Biol Chem. (1990) 265: 13308-13.
Human aldehyde dehydrogenase. Purification and characterization of a third isozyme with low Km for gamma-aminobutyraldehyde. J Biol Chem. (1989) 264: 4715-21.
A menadione-stimulated pyridine nucleotide oxidase from resting bovine neutrophil membranes. Purification, properties, and immunochemical cross-reactivity with the human neutrophil NADPH oxidase. J Biol Chem. (1988) 263: 11657-63.
Purification and characterization of two types of NADH-quinone reductase from Thermus thermophilus HB-8. Biochemistry. (1988) 27: 2008-13.
Amino acid specific ADP-ribosylation: specific NAD: arginine mono-ADP-ribosyltransferases associated with turkey erythrocyte nuclei and plasma membranes. Biochemistry. (1986) 25: 8057-62.
Purification and characterization of NADH dehydrogenase complex from Paracoccus denitrificans. Arch Biochem Biophys. (1986) 250: 302-11.
Mitochondrial nicotinamide nucleotide transhydrogenase: nonidentical modification by N,N'-dicyclohexylcarbodiimide and N-(ethoxycarbonyl)-2-ethoxy-1,2-dihydroquinoline at the NAD(H) binding site. Arch Biochem Biophys. (1985) 243: 298-304.
Purification and characterization of the secondary alcohol dehydrogenase from propane-utilizing Mycobacterium vaccae strain JOB-5. J Gen Microbiol. (1985) 131: 2901-7.
Related products:
ADP-Separopore® 4B-CL (Lyophilized) (SKU # 20181057)
AMP-Separopore® 4B-CL (Lyophilized) (SKU # 20181000)
ATP-Separopore® 4B-CL (Lyophilized) (SKU # 20181080)
ATP-Separopore® 4B-CL (SKU # 20181051)
N-Acetyl-galactosamine Separopore® 4B-CL (SKU # 20181021)
N-Acetyl-glucosamine Separopore® 4B-CL (SKU # 20181022)
GTP-Separopore® 4B-CL (SKU # 20181066)
NADP-Separopore® 4B-CL (Lyophilized) (SKU # 20181023)
Usage: bioWORLD's products are supplied for LABORATORY RESEARCH USE ONLY. The product may not be used as a drug, agricultural or pesticidal product , food additive or as a household chemical.
Hazmat Shipping: Non-hazardous
Storage: -20°C
Appearance Form: Powder
Appearance Color: Off-white
Brand Name: bioPLUS™, Separopore®
| Is Featured? | No |
|---|
Write Your Own Review