Protein A sepharose
€229.00
In stock
SKU
BT-BT002
Description: Protein A resin, Protein A agarose
Reagent: Cross-linked agarose conjugated with recombinant protein A. The protein A resin is supplied as 50% slurry in PBS/20% Ethanol.
Recommended usage:
Protein A Sepharose can be used to purify IgG antibody or recombinant protein with IgG-Fc tag. The binding capacity for the resin is about 20mg of human IgG1 antibody per mL of resin.
Vial Size: 1mL, 10mL, 50mL
Storage and Handling: Store the vial at 4°C (DO NOT FREEZE). The unopened vial is stable for twelve months when stored at 4°C.
Chemical stability: 0.5M NaOH, 6M guanidine hydrochloride, 20% ethanol, 8M urea, commonly used aqueous buffers for protein A purification
Application: Protein Purification, Immunoprecipitation
Recommended protocol: The protein A Sepharose resin can be used for purification of IgG antibody or Fc-tagged recombinant protein in the batch or column method.
Resin Preparation: thoroughly resuspend the protein A resin by gentle inversion until the resin is in uniform suspension. Apply the desired volume of the resin into the empty column and allow the beads to be settled down and liquid drained. Do not let the resin run dry. Apply at least 2 column volume (net resin volume) of PBS or PBS/0.09% NaN3 to wash off the ethanol. Now the resin is ready for use.
Binding and washing: for column purification, we recommend to run cell lysate or culture sup at the speed of 0.5-1.0mL/min. For batch purification, the washed protein A resin can be added to the cell lysate or culture sup and stir with low speed (50-70 rpm) on the stir plate or rotating or the rotator. The binding usually can be competed at room temperature for 3-5 hours or overnight at 4°C. After the batch binding, the sample with the resin can be loaded into Bio-rad Econo-column. After the resin is completely settled down (for batch purification) or after the lysate or sup is completely loaded into the column (for column purification), wash the column with PBS or PBS/0.09% NaN3 to get rid of non-specific binding. This can be monitor by Bradford method or UV monitor till it reaches the baseline
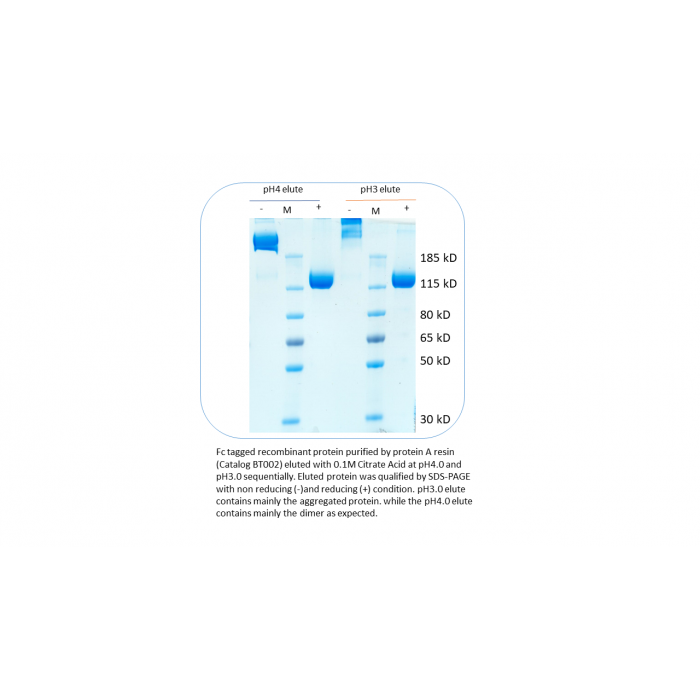
Target Protein Elution: Bound protein can be eluted with 0.1M Glycine pH2.7. Otherwise, the protein can be eluted step wise with 0.1M Citrate acid pH5, pH4 and then pH Neutralize the eluted protein with 2M Tris PH8.0 immediately (usually 0.1 volume of elution is sufficient). Usually for Fc tagged protein, we recommended to use stepwise elution and run the SDS-PAGE (reducing and non-reducing condition) and size exclusion column to check whether the aggregation status (See attached figures showing that pH3.0 elute has aggregated protein and pH4.0 elute has much less aggregate). The aggregation status of certain protein might affect its function.
Resin regeneration: the resin can be re-used for up to 10 times without significant lost in binding capacity. After each use, wash the column with 5 column volume of the elution buffer and then equilibrate the column with 5-10 column volume of PBS/0.09% sodium azide. For more thorough regeneration, the resin can be washed sequentially with 6M guanidine-HCl (2-5 column volume), 0.5M NaOH (2-5 column volume), deionized water (5-10 column volume), PBS/0.09% sodium azide or PBS/20% Ethanol (5-10 column volume). Transfer the resin into appropriate container and store it at 4°C. For long term storage we recommend storing the resin in PBS/20% Ethanol at 4°C.
Reagent: Cross-linked agarose conjugated with recombinant protein A. The protein A resin is supplied as 50% slurry in PBS/20% Ethanol.
Recommended usage:
Protein A Sepharose can be used to purify IgG antibody or recombinant protein with IgG-Fc tag. The binding capacity for the resin is about 20mg of human IgG1 antibody per mL of resin.
Vial Size: 1mL, 10mL, 50mL
Storage and Handling: Store the vial at 4°C (DO NOT FREEZE). The unopened vial is stable for twelve months when stored at 4°C.
Chemical stability: 0.5M NaOH, 6M guanidine hydrochloride, 20% ethanol, 8M urea, commonly used aqueous buffers for protein A purification
Application: Protein Purification, Immunoprecipitation
Recommended protocol: The protein A Sepharose resin can be used for purification of IgG antibody or Fc-tagged recombinant protein in the batch or column method.
Resin Preparation: thoroughly resuspend the protein A resin by gentle inversion until the resin is in uniform suspension. Apply the desired volume of the resin into the empty column and allow the beads to be settled down and liquid drained. Do not let the resin run dry. Apply at least 2 column volume (net resin volume) of PBS or PBS/0.09% NaN3 to wash off the ethanol. Now the resin is ready for use.
Binding and washing: for column purification, we recommend to run cell lysate or culture sup at the speed of 0.5-1.0mL/min. For batch purification, the washed protein A resin can be added to the cell lysate or culture sup and stir with low speed (50-70 rpm) on the stir plate or rotating or the rotator. The binding usually can be competed at room temperature for 3-5 hours or overnight at 4°C. After the batch binding, the sample with the resin can be loaded into Bio-rad Econo-column. After the resin is completely settled down (for batch purification) or after the lysate or sup is completely loaded into the column (for column purification), wash the column with PBS or PBS/0.09% NaN3 to get rid of non-specific binding. This can be monitor by Bradford method or UV monitor till it reaches the baseline
Target Protein Elution: Bound protein can be eluted with 0.1M Glycine pH2.7. Otherwise, the protein can be eluted step wise with 0.1M Citrate acid pH5, pH4 and then pH Neutralize the eluted protein with 2M Tris PH8.0 immediately (usually 0.1 volume of elution is sufficient). Usually for Fc tagged protein, we recommended to use stepwise elution and run the SDS-PAGE (reducing and non-reducing condition) and size exclusion column to check whether the aggregation status (See attached figures showing that pH3.0 elute has aggregated protein and pH4.0 elute has much less aggregate). The aggregation status of certain protein might affect its function.
Resin regeneration: the resin can be re-used for up to 10 times without significant lost in binding capacity. After each use, wash the column with 5 column volume of the elution buffer and then equilibrate the column with 5-10 column volume of PBS/0.09% sodium azide. For more thorough regeneration, the resin can be washed sequentially with 6M guanidine-HCl (2-5 column volume), 0.5M NaOH (2-5 column volume), deionized water (5-10 column volume), PBS/0.09% sodium azide or PBS/20% Ethanol (5-10 column volume). Transfer the resin into appropriate container and store it at 4°C. For long term storage we recommend storing the resin in PBS/20% Ethanol at 4°C.
| Is Featured? | No |
|---|
Write Your Own Review